Description
Here's a detailed product description for medical implants, suitable for a professional audience, formatted in Markdown.
Revolutionary Medical Implants: Restoring Function. Enhancing Life.
Product Overview
Our comprehensive range of medical implants is meticulously engineered to meet the highest standards of safety, efficacy, and durability. Designed to integrate seamlessly with the human body, these advanced solutions are pivotal in restoring function, alleviating pain, and significantly improving the quality of life for patients across various medical disciplines. Leveraging cutting-edge materials and precision manufacturing, we are committed to pushing the boundaries of medical technology to support optimal patient outcomes.
Key Features & Innovations
- Superior Biocompatibility: Crafted from medical-grade materials such as titanium alloys, PEEK, ceramic composites, and medical-grade stainless steel, ensuring minimal immunological response and excellent integration with surrounding tissues.
- Precision Engineering & Design: Utilizing advanced CAD/CAM software and 3D printing technologies for anatomically correct, patient-specific, and highly precise implant geometries that optimize fit and performance.
- Enhanced Surface Technologies: Featuring state-of-the-art surface treatments (e.g., plasma spray coatings, anodic oxidation, hydroxyapatite coatings, micro-texturing) to promote faster osseointegration, tissue adhesion, and reduce bacterial colonization.
- Exceptional Durability & Longevity: Rigorously tested for fatigue, wear resistance, and corrosion, ensuring long-term structural integrity and performance under physiological loads.
- Comprehensive Portfolio: A broad spectrum of implants catering to diverse medical specialties, available in various sizes, shapes, and configurations to address a wide range of patient anatomies and clinical indications.
- Sterile & Ready-to-Use: Each implant is terminally sterilized and individually packaged to ensure asepsis and immediate availability for surgical procedures.
- Traceability & Quality Assurance: Every implant is accompanied by comprehensive documentation, ensuring full traceability from raw material to patient, adhering to stringent quality control protocols.
Applications & Clinical Indications
Our medical implants are indicated for a wide array of reconstructive, reparative, and supportive procedures, including but not limited to:
- Orthopedic Implants:
- Joint Arthroplasty (Hip, Knee, Shoulder, Ankle replacements)
- Spinal Fusion Devices (Interbody cages, pedicle screws, rods)
- Trauma Fixation (Plates, screws, rods, pins for fractures)
- Sports Medicine (Ligament reconstruction anchors, tendon repair devices)
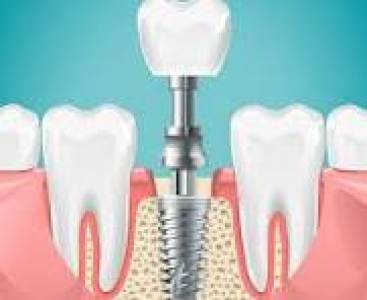
- Dental Implants:
- Endosseous implants for prosthetic tooth replacement
- Abutments and restorative components
- Maxillofacial reconstruction plates and screws
- Craniofacial Implants:
- Reconstruction plates and meshes for skull and facial bone defects
- Orbital floor repair
- Cardiovascular Implants: (e.g., Pacemaker leads, vascular stents - Note: While within the broader implant category, specific product lines would focus on these. This description remains general.)
- Neurological Implants: (e.g., Deep brain stimulation electrodes, cranial access ports - Similar to cardiovascular, very specialized.)
- Soft Tissue Repair:
- Hernia repair meshes
- Breast implants (reconstructive and aesthetic)
Advantages for Healthcare Professionals & Patients
For Surgeons & Medical Teams:
- Predictable Outcomes: Reliable design and proven materials contribute to consistent and successful surgical results.
- Ease of Implantation: Ergonomic designs and compatible instrumentation streamline surgical procedures.
- Reduced Complications: High biocompatibility and advanced surface features minimize the risk of infection and adverse reactions.
- Comprehensive Support: Access to technical documentation, clinical studies, and dedicated professional support.
For Patients:
- Improved Quality of Life: Restoration of mobility, alleviation of pain, and functional improvement enable a return to daily activities.
- Long-Term Stability: Designed for enduring performance, reducing the need for revision surgeries.
- Enhanced Comfort: Anatomically contoured designs and smooth finishes contribute to better integration and patient comfort.
- Faster Recovery: Materials and designs optimized for rapid biological integration may support quicker rehabilitation.
Technical Specifications (Illustrative Examples)
- Materials: Titanium (Ti-6Al-4V ELI), PEEK (Polyetheretherketone), CoCrMo (Cobalt-Chromium-Molybdenum) alloys, UHMWPE (Ultra-High Molecular Weight Polyethylene), Zirconia ceramics.
- Surface Treatment Options: Anodization, Hydroxyapatite (HA) coating, Plasma spray, Acid-etched, Sandblasted, Porous structures.
- Sizes & Configurations: Wide range of diameters, lengths, angulations, and custom patient-specific options available upon request.
- Sterilization Method: Gamma irradiation or Ethylene Oxide (ETO) sterilization, ensuring a minimum Sterility Assurance Level (SAL) of 10^-6.
- Packaging: Double-pouched, sterile barrier system with clear labeling and unique device identification (UDI).
Quality Assurance & Regulatory Compliance
Our manufacturing processes adhere to the strictest international standards for medical devices. We maintain certifications including:
- ISO 13485: Quality Management System for Medical Devices
- FDA Compliance: Registered and listed with the U.S. Food and Drug Administration (FDA)
- CE Mark: Conforming to European medical device directives (MDR/MDD)
- Rigorous Testing: Comprehensive biocompatibility, mechanical, and fatigue testing in accordance with ASTM and ISO standards.
Ordering Information & Support
For detailed product catalogs, clinical data, pricing, or to schedule a consultation with our medical device specialists, please contact us:
Our dedicated team is ready to provide comprehensive support, including product training, technical assistance, and logistical coordination to meet your clinical needs.
Disclaimer
This product description is intended for healthcare professionals only. The use of medical implants requires appropriate medical training and judgment. Specific indications, contraindications, warnings, and precautions can be found in the individual product's Instructions for Use (IFU). Always refer to the IFU prior to use.