Description
Here's a detailed product description for "Implants" in Markdown format, designed to be comprehensive and professional for a B2B audience (medical professionals, procurement, distributors).
Implants: Restoring Function, Enhancing Life
Product Name: Comprehensive Medical Implant Solutions
Description: Implants represent a cornerstone of modern medicine, offering innovative and life-changing solutions to restore, replace, or enhance compromised biological structures. From life-saving cardiac devices to mobility-restoring orthopedic components and aesthetically transformative dental solutions, our comprehensive range of implants is engineered for unparalleled precision, durability, and superior patient outcomes.
Developed through extensive research and leveraging cutting-edge materials science, our implant portfolio is designed to integrate seamlessly with the human body. These devices are crucial for improving patient quality of life, restoring essential bodily functions, alleviating pain, and providing lasting therapeutic benefits.
Key Features:
- Advanced Biocompatible Materials: Utilizing medical-grade titanium alloys, cobalt-chromium, stainless steel, ceramics, specialized polymers (e.g., PEEK), and medical-grade silicone for optimal biological integration and minimal adverse reactions.
- Precision Engineering & Manufacturing: Employing state-of-the-art CAD/CAM, CNC machining, and additive manufacturing (3D printing) to ensure exact anatomical fit and superior mechanical properties.
- Broad Application Spectrum: A diverse portfolio addressing multiple medical specialties and patient needs.
- Long-Term Durability & Reliability: Engineered for exceptional wear resistance, fatigue strength, and structural integrity, ensuring extended functional lifespan.
- Stringent Quality Control: Adherence to the highest international quality standards (e.g., ISO 13485) throughout design, production, and sterilization processes.
- Customizable Solutions: Options for various sizes, configurations, and patient-specific designs to accommodate diverse anatomies and clinical requirements.
- Regulatory Compliance: Fully compliant with global regulatory bodies, including FDA (US), CE Marking (EU), and other national health authority approvals.
Types & Applications:
Our extensive range of implantable devices includes, but is not limited to:
- Orthopedic Implants:
- Total Joint Replacements: Hip (femoral stems, acetabular cups), Knee (femoral, tibial, patellar components), Shoulder (humeral heads, glenoid components).
- Spinal Implants: Interbody fusion cages (TLIF, PLIF, ALIF), pedicle screw systems, rods, plates, artificial discs.
- Trauma Implants: Bone plates, screws, nails, intramedullary rods for fracture fixation.
- Sports Medicine Implants: Anchors, screws, and allografts/autograft components for ligament and tendon repair.
- Bone Grafts & Substitutes: Synthetic and allograft options for bone regeneration and fusion.
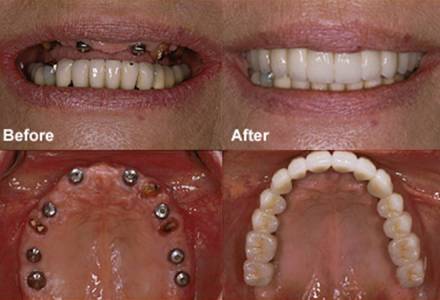
- Dental Implants:
- Root-Form Implants: Titanium or zirconia posts surgically placed into the jawbone.
- Abutments: Connectors between the implant and prosthetic crown, bridge, or denture.
- Restorative Components: Crowns, bridges, and implant-supported dentures.
- Guided Surgery Kits: For precise implant placement.
- Cardiovascular Implants:
- Pacemakers & Defibrillators (ICDs): For regulating heart rhythm and preventing sudden cardiac arrest.
- Stents: Coronary and peripheral vascular stents for opening narrowed arteries.
- Artificial Heart Valves: Mechanical and tissue valves for valve replacement.
- Vascular Grafts: For bypass surgery or aneurysm repair.
- Neurological Implants:
- Deep Brain Stimulators (DBS): For treating Parkinson's disease, essential tremor, and other movement disorders.
- Spinal Cord Stimulators (SCS): For chronic pain management.
- Cochlear Implants: For severe-to-profound hearing loss.
- Vagal Nerve Stimulators (VNS): For epilepsy and depression.
- Ocular Implants:
- Intraocular Lenses (IOLs): Monofocal, multifocal, and toric lenses for cataract surgery.
- Glaucoma Drainage Devices: For managing intraocular pressure.
- Cosmetic & Reconstructive Implants:
- Breast Implants: Saline and silicone options for augmentation and reconstruction.
- Facial Implants: Chin, cheek, and jaw implants for aesthetic enhancement or reconstruction.
- Tissue Expanders: For gradual skin and tissue growth in reconstructive procedures.
- Surgical Mesh:
- Hernia Repair Mesh: Synthetic and biological meshes for reinforcement of abdominal wall defects.
- Pelvic Floor Reconstruction Mesh: For stress urinary incontinence and pelvic organ prolapse.
Core Benefits for Healthcare Providers & Patients:
- Restored Function & Mobility: Enable patients to regain lost capabilities, from walking to hearing.
- Significant Pain Relief: Alleviate chronic pain associated with degenerative conditions, injuries, or neurological disorders.
- Enhanced Quality of Life: Improve daily activities, self-esteem, independence, and overall well-being.
- Long-Term Therapeutic Solutions: Designed for years, often decades, of reliable and effective performance, reducing the need for repeat interventions.
- Reduced Complications: Advanced materials and design minimize risks of infection, rejection, and mechanical failure.
- Optimized Surgical Outcomes: User-friendly designs and comprehensive support facilitate efficient and successful surgical procedures.
Materials & Advanced Technology:
Our implants are manufactured using a selection of highly tested and approved medical-grade materials, chosen for their superior biocompatibility, strength, and durability. These include:
- Metals: Titanium and its alloys (Ti-6Al-4V), Cobalt-Chromium alloys, Stainless Steel (316L).
- Polymers: PEEK (Polyetheretherketone), UHMWPE (Ultra-High Molecular Weight Polyethylene), Silicone.
- Ceramics: Alumina, Zirconia.
Manufacturing processes incorporate advanced techniques such as:
- Precision CNC machining
- Additive Manufacturing (3D Printing) for complex geometries and custom implants
- Surface treatments (e.g., porous coatings for osseointegration, plasma-spraying, anodization)
- Sterile packaging in controlled environments (Class 10,000 cleanrooms)
- Non-destructive testing (NDT) to ensure structural integrity
Quality Assurance & Regulatory Compliance:
Every implant undergoes rigorous quality control at each stage of design, manufacturing, and packaging. Our commitment to excellence ensures products meet and exceed the highest international regulatory standards:
- ISO 13485 Certified: Quality management system for medical devices.
- FDA 510(k) Cleared / PMA Approved: For relevant products in the United States.
- CE Marked: Conforming to European Union health, safety, and environmental protection standards.
- Country-Specific Health Authority Approvals: Compliance with regulations in target markets worldwide.
- Full Traceability: Each implant is traceable from raw material to final sterile product.
Indications for Use:
Specific indications for use vary significantly by implant type and are detailed in their respective product inserts. Generally, implants are indicated for patients suffering from disease, trauma, or congenital conditions requiring structural replacement, functional augmentation, or therapeutic intervention where non-invasive treatments are insufficient or inappropriate.
Installation, Training & Support:
Our implants are designed for seamless integration with established surgical techniques. We provide:
- Comprehensive Technical Documentation: Detailed instructions for use, surgical manuals, and clinical data.
- Professional Training Programs: Educational resources and workshops for surgeons and clinical staff.
- Dedicated Clinical Support: On-call specialists to assist during surgical procedures and post-operative care.
Maintenance & Longevity:
While implants are designed for exceptional durability and long-term performance, their longevity can be influenced by patient factors (age, activity level, health status), lifestyle choices, and adherence to post-operative care protocols. Regular follow-ups with healthcare providers are essential for monitoring implant performance and ensuring long-term success.
Target Audience:
- Hospitals and surgical centers
- Specialized medical clinics (Orthopedic, Dental, Cardiology, Neurology, Ophthalmology)
- Government health organizations
- Medical device distributors and wholesalers
- Surgeons and medical practitioners seeking high-quality implantable solutions
Ordering Information & Contact:
For detailed product specifications, clinical data, pricing, or to schedule a consultation with our medical device specialists, please contact our sales team. We are committed to partnering with healthcare professionals to improve patient lives.
[Your Company Name/Logo] [Your Company Website] [Your Company Email] [Your Company Phone Number] [Your Company Address]
Disclaimer: Implants are prescription medical devices. Their use, selection, and surgical implantation require the judgment and skill of a qualified healthcare professional. Risks and benefits should be thoroughly discussed with patients prior to any procedure. This product description is for informational purposes only and does not constitute medical advice or a recommendation for a specific product or treatment.